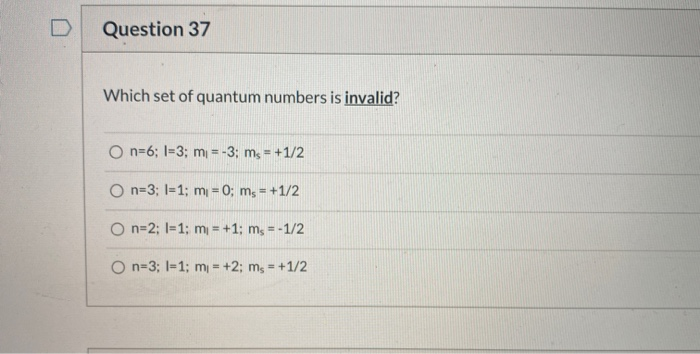
In quantum mechanics, quantum numbers are used to describe the properties of atomic orbitals and the electrons within them. Each electron in an atom is defined by a set of four quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (mₗ), and the spin quantum number (mₛ). These quantum numbers follow specific rules and restrictions.
Understanding which set of quantum numbers is invalid is important for properly describing electron configurations. Let’s explore the valid and invalid combinations of quantum numbers.
The Four Quantum Numbers
1. Principal Quantum Number (n)
The principal quantum number describes the energy level of an electron and can take any positive integer value (1, 2, 3, …).
2. Angular Momentum Quantum Number (l)
The angular momentum quantum number defines the shape of the orbital. It can take integer values from 0 to (n-1) for each value of n.
- For n = 1, l can only be 0.
- For n = 2, l can be 0 or 1.
- For n = 3, l can be 0, 1, or 2, and so on.
3. Magnetic Quantum Number (mₗ)
The magnetic quantum number determines the orientation of the orbital in space. For a given value of l, mₗ can take integer values ranging from -l to +l (including zero).
4. Spin Quantum Number (mₛ)
The spin quantum number specifies the spin orientation of the electron. It can take values of +1/2 or -1/2.
Conditions for Valid Quantum Numbers
- The principal quantum number n must be a positive integer.
- The angular momentum quantum number l must satisfy 0 ≤ l < n.
- The magnetic quantum number mₗ must satisfy -l ≤ mₗ ≤ +l.
- The spin quantum number mₛ must be +1/2 or -1/2.
Examples of Invalid Quantum Numbers
Example 1: n = 2, l = 2, mₗ = 0, mₛ = +1/2
- Invalid because the value of l cannot be equal to n. Since n = 2, l must be 0 or 1, not 2.
Example 2: n = 3, l = 2, mₗ = +3, mₛ = -1/2
- Invalid because the value of mₗ must lie between -l and +l. For l = 2, the allowed values of mₗ are -2, -1, 0, 1, 2, but +3 is outside this range.
Example 3: n = 1, l = 1, mₗ = 0, mₛ = +1/2
- Invalid because l must be less than n. Since n = 1, l must be 0. It cannot be 1.
Conclusion
When determining the validity of a set of quantum numbers, it’s essential to adhere to the rules governing the values of n, l, mₗ, and mₛ. Sets that violate these rules are considered invalid. Understanding these conditions will help you properly interpret and apply quantum numbers in atomic theory and chemistry.